Strontium Hydroxide Formula
- Glucoside-Based Foaming Cleansers in Dermatological Formulations and Advanced Surfactant Science - April 1, 2025
- Copper II Chloride Molecular Composition and Its Critical Role in Advanced Electrochemical Applications - April 1, 2025
- Pyridine in Pharmaceutical Science and Synthesis of Medicinal Compounds - April 1, 2025

Strontium Hydroxide FAQs: Everything You Need to Know
Strontium Hydroxide Formula Introduction:
Strontium hydroxide is a compound that holds both industrial and scientific significance. As with any chemical, it raises questions and sparks curiosity. This blog post will delve into frequently asked questions about strontium hydroxide It will provide comprehensive and insightful answers to expand your understanding of this compound. From its Strontium Hydroxide solubility in water to its chemical formula for strontium hydroxide and nature,
What is strontium hydroxide, and how is it synthesized?
Strontium hydroxide is a chemical compound comprising one strontium atom and two hydroxide groups. It is a white, crystalline powder with a high alkalinity and can react with acids to form salts. One way to synthesize strontium hydroxide is by mixing a strontium salt, such as strontium nitrate, with a strong base, such as sodium hydroxide or potassium hydroxide. This will cause strontium hydroxide to precipitate out of the solution, which can then be filtered, washed, and dried. Another way to synthesize strontium hydroxide is by exposing strontium metal to water, which will produce strontium hydroxide and hydrogen gas. Strontium hydroxide can exist in different forms depending on the amount of water it contains, such as anhydrous, monohydrate, or octahydrate.
Is strontium hydroxide soluble in water?
Strontium hydroxide is indeed soluble in water, although its Strontium Hydroxide solubility is relatively low compared to other hydroxides. At room temperature, approximately 9 grams of strontium hydroxide can dissolve in 100 milliliters of water. However, as the temperature rises, the solubility increases. It is important to note that the solution of strontium hydroxide in water is highly alkaline due to its strong basic properties.
Is strontium hydroxide a strong base?
Yes, strontium hydroxide is classified as a strong base. When dissolved in water, it readily dissociates into ions, releasing hydroxide ions (OH-) into the solution. These hydroxide ions are responsible for the alkaline nature of the resulting solution. The strong basicity of strontium hydroxide makes it an essential component in various applications, including as a catalyst and in producing other chemicals.
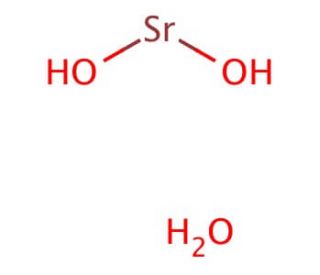
What is the chemical formula for strontium hydroxide?
The chemical formula for strontium hydroxide is Sr(OH)2. One strontium atom (Sr) is bonded with two hydroxide ions (OH-). The strontium hydroxide formula represents the elements’ ratio and their respective charges in the compound.
Is strontium hydroxide an ionic or covalent compound?
Strontium hydroxide is an ionic compound. It forms through the electrostatic attraction between the positively charged strontium ions (Sr2+) and the negatively charged hydroxide ions (OH-) present in the compound. The ionic nature of strontium hydroxide contributes to its solubility in water and its ability to conduct electricity when dissolved.
Is strontium hydroxide soluble?
Yes, strontium hydroxide is soluble in water, although its solubility is limited. As mentioned earlier, approximately 9 grams of strontium hydroxide can dissolve at room temperature in 100 millilitres of water. However, the solubility of Strontium Hydroxide increases with higher temperatures. Handling strontium hydroxide cautiously is essential, as it is highly alkaline and can cause skin and eye irritation.
What are the common applications of strontium hydroxide?
Strontium hydroxide finds applications in various industries and scientific research. Some of the common strontium hydroxide uses include:
- Manufacturing of pyrotechnic materials: Strontium hydroxide is a key ingredient in producing red-colored flame fireworks because it emits a vibrant red color when burned.
- Analytical chemistry: Strontium hydroxide is used in the laboratory for titrations, pH control, and precipitation reactions.
- Catalyst in organic synthesis: It catalyzes several chemical reactions, such as esterification and transesterification processes.
- Water treatment: Strontium hydroxide is used in water treatment processes to remove unwanted impurities and adjust pH levels.
- Production of pigments: Strontium hydroxide is crucial in manufacturing pigments, including those used in ceramics, paints, and dyes.

What are the hazards and safety precautions of strontium hydroxide?
Strontium hydroxide is a chemical compound that can cause severe skin burns and eye damage if it comes in contact with the skin or eyes. It may also cause respiratory irritation if inhaled. Therefore, it is important to follow some safety precautions when handling this substance.
Some of the safety precautions are:
- Wear suitable protective clothing, gloves, eye protection and face protection.
- Avoid contact with skin and eyes, and avoid formation of dust and aerosols.
- Use non-sparking tools and prevent fire caused by electrostatic discharge steam.
- Do not breathe dust/fume/gas/mist/vapors/spray.
- Use only outdoors or in a well-ventilated area.
- Wash face, hands and any exposed skin thoroughly after handling.
- Store in a locked container in a cool, dry and well-ventilated place.
If exposed to strontium hydroxide, seek immediate medical attention and follow these steps:
- If inhaled, remove it to fresh air and keep it at rest. If it is not breathing, give it artificial respiration.
- If on skin or hair, take off contaminated clothing and shoes immediately. Rinse skin with water or shower. Wash contaminated clothing before reuse.
- If in eyes, rinse cautiously with water for several minutes. If contact lenses are present and easy to remove, continue rinsing
- If swallowed, rinse your mouth. Do not induce vomiting.
What are the US’s current regulations and standards for strontium hydroxide in the hydroxide?
According to the U.S. Environmental Protection Agency (EPA), strontium hydroxide is classified as a hazardous substance under the Clean Water Act, with a reportable quantity of 1,000 pounds (454 kilograms). Any release of this amount or more to the environment must be reported to the National Response Center. The Occupational Safety and Health Administration (OSHA) also considers strontium hydroxide hazardous, and upon exposure, it may cause severe skin burns, eye damage, and respiratory irritation.
Strontium hydroxide is regulated by various federal and state agencies, such as the EPA, OSHA, the Department of Transportation (DOT), and the Food and Drug Administration (FDA). Different standards and requirements may apply depending on the use and handling of strontium hydroxide For example, strontium hydroxide used in food processing must comply with the FDA regulations for food additives, while strontium hydroxide transported by rail or road must follow the DOT regulations for hazardous materials.
Conclusion:
Strontium hydroxide is a compound that possesses fascinating properties and applications. From its strontium hydroxide solubility in water to its status as a strong base, understanding the fundamental aspects of this compound is essential. Armed with knowledge about its chemical formula, for strontium hydroxide ionic nature, and common uses, we can appreciate the diverse roles it plays in various industries. Whether you’re interested in pyrotechnics, analytical chemistry, or water treatment, strontium hydroxide offers intriguing possibilities. So, next time you encounter a strontium hydroxide compound, you’ll understand its characteristics and significance more deeply. At Sarchem Labs, we provide custom chemical solutions as per your requirements.
If you are looking to purchase Strontium Hydroxide then, you can check out our product:
Strontium hydroxide octahydrate | 1311-10-0
Specifications Assay Percent Range 99% (metals basis) strontium hydroxide Solubility Information Soluble in hot water and acid. Insoluble in acetone. Strontium hydroxide Formula Weight 265.76 (121.60 Anhydrous) Physical Form Crystalline Density 1.9 Sensitivity Air sensitive; Hygroscopic Melting Point 100°C-8H2O Chemical Name or Material Strontium hydroxide octahydrate