Phosphorus Trichloride Formula
- Glucoside-Based Foaming Cleansers in Dermatological Formulations and Advanced Surfactant Science - April 1, 2025
- Copper II Chloride Molecular Composition and Its Critical Role in Advanced Electrochemical Applications - April 1, 2025
- Pyridine in Pharmaceutical Science and Synthesis of Medicinal Compounds - April 1, 2025
Unlocking the Secrets of Phosphorus Trichloride: Chemistry, Applications, and Molecule Magic
What is Phosphorus Trichloride?
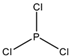
Phosphorus Trichloride, denoted by the chemical formula PCl3, is an inorganic compound composed of one phosphorus atom and three chlorine atoms. It is a colorless or slightly yellow fuming liquid with a pungent, unpleasant odor. This compound is not naturally occurring and is synthesized from organic substances2. It’s an important industrial chemical that manufactures phosphites and other organophosphorus compounds. However, it’s highly reactive and toxic, reacting violently with water to release hydrogen chloride. Due to its corrosive nature, it should not come into direct contact with eyes or skin or be inhaled or ingested.
Lat Workouts: 5 Back Exercises For Strong Wide Lats steroidly Incline Hammer Curls | Exercise Videos & Guides | Bodybuilding.comApplications: What is Phosphorus Trichloride Used for?
Phosphorus trichloride is an inorganic compound that is made up of one phosphorus and three chlorine atoms. It is a very reactive and volatile compound that is poisonous. It is used to manufacture other necessary chemicals, such as phosphate ester insecticides, surfactants, gasoline additives, plasticizers, dyestuffs, textile finishing agents, antiseptics, medicinal products, and flame retardants. It is also used as an essential reagent in organic chemistry to replace the hydroxyl group with a chlorine atom. Phosphorus trichloride is obtained from the synthesis of organic substances and not naturally occuring. It should not come in direct contact with eyes and skin or be inhaled or ingested.
Chemical Properties: Is Phosphorus Trichloride Ionic or Covalent?
Phosphorus trichloride is a binary compound consisting of phosphorus and chlorine atoms. It is a covalent compound because the high ionization energy of phosphorus does not favor the formation of a P3+ ion. Instead, the atoms are bound by shared electrons, with each bond containing one electron from the phosphorus atom and another electron from the chlorine atom. The electronegativity values of phosphorus and chlorine are similar, which also favors the formation of a covalent bond. Phosphorus trichloride is used in various industrial applications, such as manufacturing phosphate ester insecticides, surfactants, gasoline additives, plasticizers, dyestuffs, textile finishing agents, antiseptics, medicinal products, and flame retardants. It is also an important reagent in organic chemistry to replace the hydroxyl group with a chlorine atom. However, it is a very reactive and volatile compound that is poisonous and should not come in direct contact with eyes and skin or be directly inhaled or ingested.
Decoding the Chemical Formula for Phosphorus Trichloride
The Significance of the Phosphorus Trichloride Formula
Phosphorus trichloride is a chemical compound with the formula PCl3. It is a colorless liquid with a pungent odor and reacts violently with water. The formula PCl3 tells us that one molecule of phosphorus trichloride consists of one atom of phosphorus and three atoms of chlorine. The procedure also indicates the type of bonding and the molecule’s shape.
Interpreting the Chemical Formula
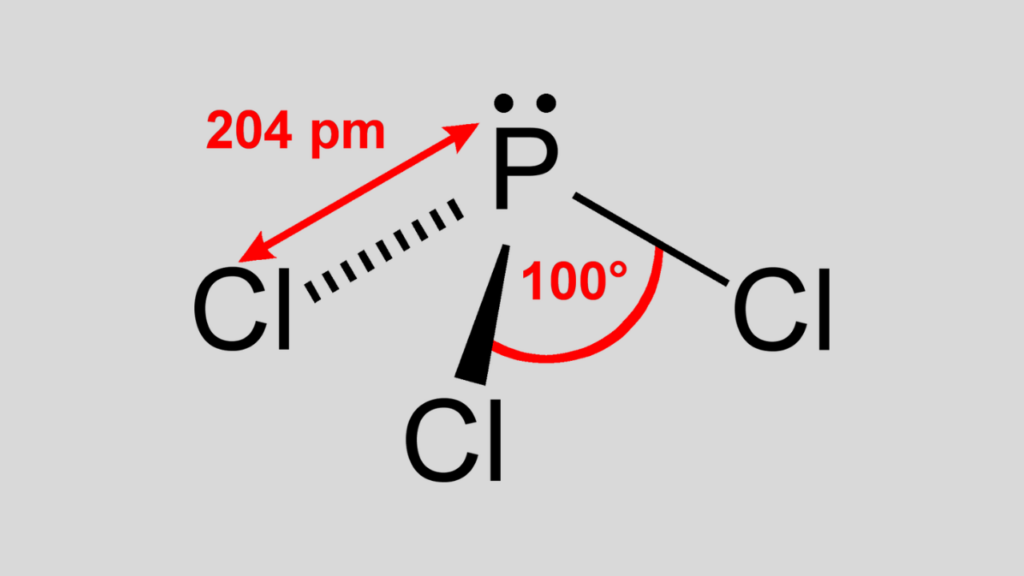
We need to know each element’s valence electrons and electronegativity to interpret the chemical formula for phosphorus trichloride (PCl3).Valence electrons are the outermost electrons that are involved in chemical bonding. Electronegativity measures how strongly an atom attracts electrons in a bond. Phosphorus has five valence electrons, and chlorine has seven. Phosphorus is less electronegative than chlorine, meaning chlorine will pull the electrons more toward itself in a bond. Therefore, phosphorus trichloride has polar covalent bonds, where the electrons are shared unequally between the atoms. The molecule’s shape is trigonal pyramidal, where the phosphorus atom is at the apex, and the three chlorine atoms are at the corners of a triangular base. The bond angle between the chlorine atoms is 107.8 degrees.
Ionic or Covalent: The Bonding in Phosphorus Trichloride
It is a covalent compound because the high ionization energy of phosphorus does not favor the formation of a P3+ ion. Instead, the atoms are bound by shared electrons, with each bond containing one electron from the phosphorus atom and another electron from the chlorine atom. The electronegativity values of phosphorus and chlorine are similar, which also favors the formation of a covalent bond. Phosphorus trichloride is used in various industrial applications, such as manufacturing phosphate ester insecticides, surfactants, gasoline additives, plasticizers, dyestuffs, textile finishing agents, antiseptics, medicinal products, and flame retardants. It is also an important reagent in organic chemistry to replace the hydroxyl group with a chlorine atom.
Conclusion
Phosphorus Trichloride (PCl3) is an essential inorganic compound consisting of one phosphorus atom and three chlorine atoms. It is a colorless to slightly yellow fuming liquid with a pungent, unpleasant odor. PCl3 is not naturally occurring and is synthesized from organic substances. It holds significant industrial importance, manufacturing various chemicals, including phosphate ester insecticides, surfactants, gasoline additives, plasticizers, dyestuffs, textile finishing agents, antiseptics, medicinal products, and flame retardants. Additionally, it serves as a crucial reagent in organic chemistry for replacing hydroxyl groups with chlorine atoms.
Regarding its chemical properties, Phosphorus Trichloride exhibits covalent bonding due to the similar electronegativities of phosphorus and chlorine. This results in shared electrons in its bonds, creating polar covalent bonds where electrons are shared unevenly. The molecule’s shape is trigonal pyramidal, with a bond angle between chlorine atoms measuring 107.8 degrees.
In conclusion, Phosphorus Trichloride is a versatile compound with widespread industrial applications, characterized by its reactivity, toxicity, and covalent bonding nature. Careful handling is essential due to its corrosive properties, making direct contact with eyes, skin, inhalation, or ingestion highly hazardous.
Sarchem Labs, a leading provider of chemical synthesis solutions and a global distributor of specialty chemicals, plays a crucial role in the safe and effective use of such compounds. Over three decades of experience, Sarchem Labs has delivered best-in-class chemical solutions to a diverse client base. Their expert chemistry team is dedicated to customer service and innovation², ensuring that compounds like Phosphorus Trichloride are utilized effectively and responsibly in various industries.
Frequently Asked Questions
What is Phosphorus Trichloride?
Phosphorus Trichloride, denoted by the chemical formula PCl3, is an inorganic compound composed of one phosphorus atom and three chlorine atoms. It is a colorless or slightly yellow fuming liquid with a pungent odor.What are the applications of Phosphorus Trichloride?
Phosphorus Trichloride is used to manufacture various chemicals, including phosphate ester insecticides, surfactants, gasoline additives, plasticizers, dyestuffs, textile finishing agents, antiseptics, medicinal products, and flame retardants. It’s also used as a reagent in organic chemistry reactions.Is Phosphorus Trichloride a covalent or ionic bond?
Phosphorus Trichloride is a covalent bond. It forms covalent bonds because the ionization energy of phosphorus does not favor the formation of P3+ ions, and the electronegativity values of phosphorus and chlorine are similar.What are the chemical properties of Phosphorus Trichloride?
Phosphorus Trichloride is highly reactive and toxic. PCl3 violently reacts with water to release hydrogen chloride, and it should not be in direct contact with the eyes or skin, inhaled, or ingested.How is Phosphorus Trichloride obtained?
Phosphorus Trichloride is obtained through the synthesis of organic substances and is not found naturally in its pure form.What is the chemical formula for Phosphorus Trichloride?
The chemical formula for Phosphorus Trichloride is PCl3, indicating one phosphorus atom and three chlorine atoms in each molecule.What is the shape of the Phosphorus Trichloride molecule?
The molecule has a trigonal pyramidal shape, with the phosphorus atom at the apex and three chlorine atoms at the corners of a triangular base. The bond angle between the chlorine atoms is approximately 107.8 degrees.Why is Phosphorus Trichloride considered toxic and corrosive?
Phosphorus Trichloride is toxic because of its chemical reactivity and can release hydrogen chloride upon contact with water. It is also corrosive, and trained professionals should handle the material carefully to avoid direct contact with skin, eyes, and inhalation.What are some common industrial uses of Phosphorus Trichloride?
It is used to manufacture various industrial products, including flame retardants, plasticizers, and medicinal products. It is also employed as a reagent in organic chemistry reactions.Is Phosphorus Trichloride polar or nonpolar?
Phosphorus Trichloride (PCl3) is a polar molecule due to its tetrahedral geometrical shape and a lone pair on the Phosphorus atom. The difference in electronegativity between Chlorine (3.16) and Phosphrus (2.19) atoms results in unequal sharing of electrons, which develops positive and negative poles across the molecule.If you are looking to buy Phosphorus Trichloride, then you may check out our product
Phosphorus trichloride | 7719-12-2
Author Recent Posts Sarchem LabsGeneral Manager at Sarchem Laboratories, Inc.Sarchem Laboratories offers a combined 60 years of professional experience in the field of pharmaceutical intermediaries, specializing in custom synthesis. Founded in 1984 as a research center focused on anti-viral drugs, Sarchem Laboratories has expanded over the years to the adapting needs of the pharmaceutical, nutraceutical…